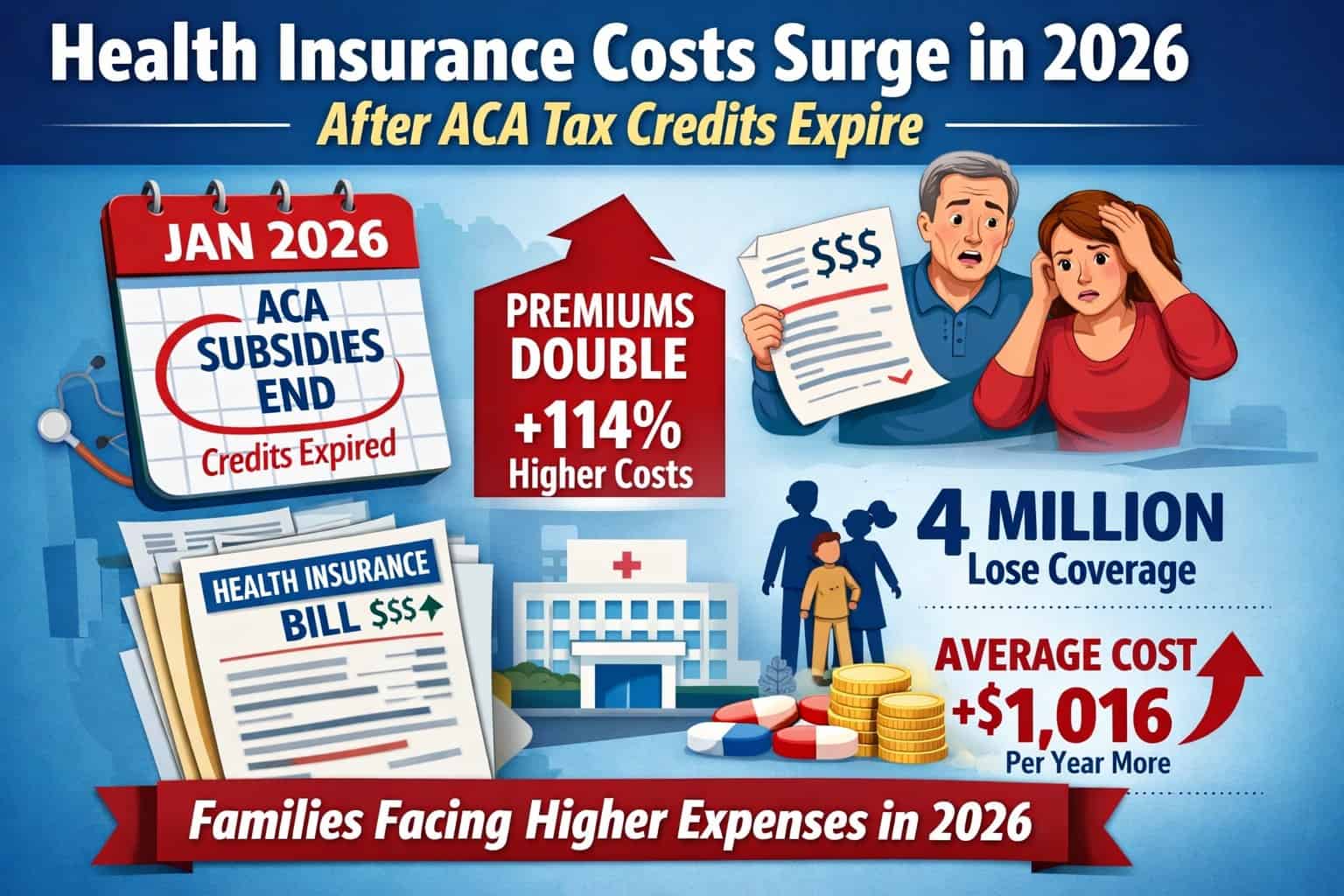
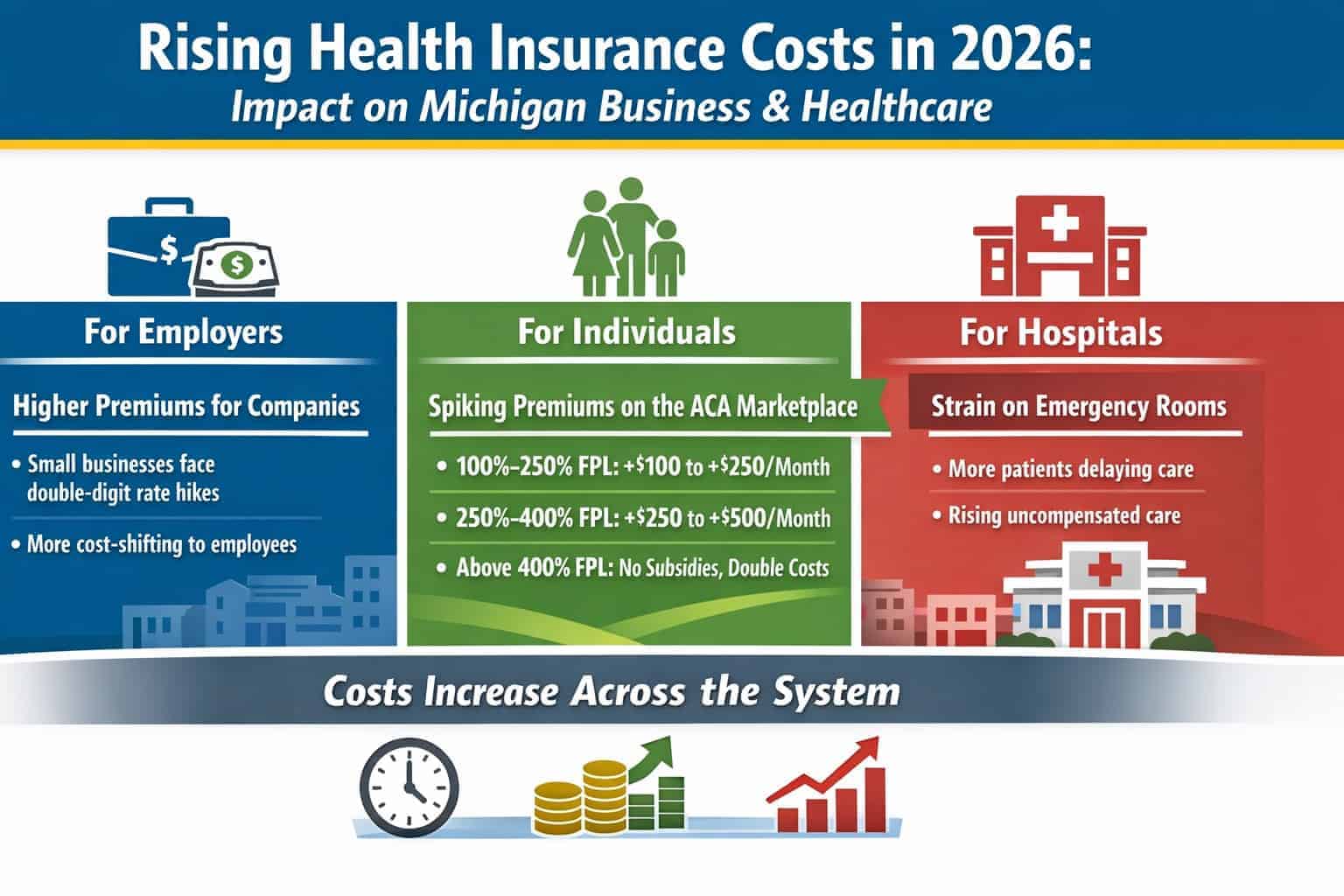
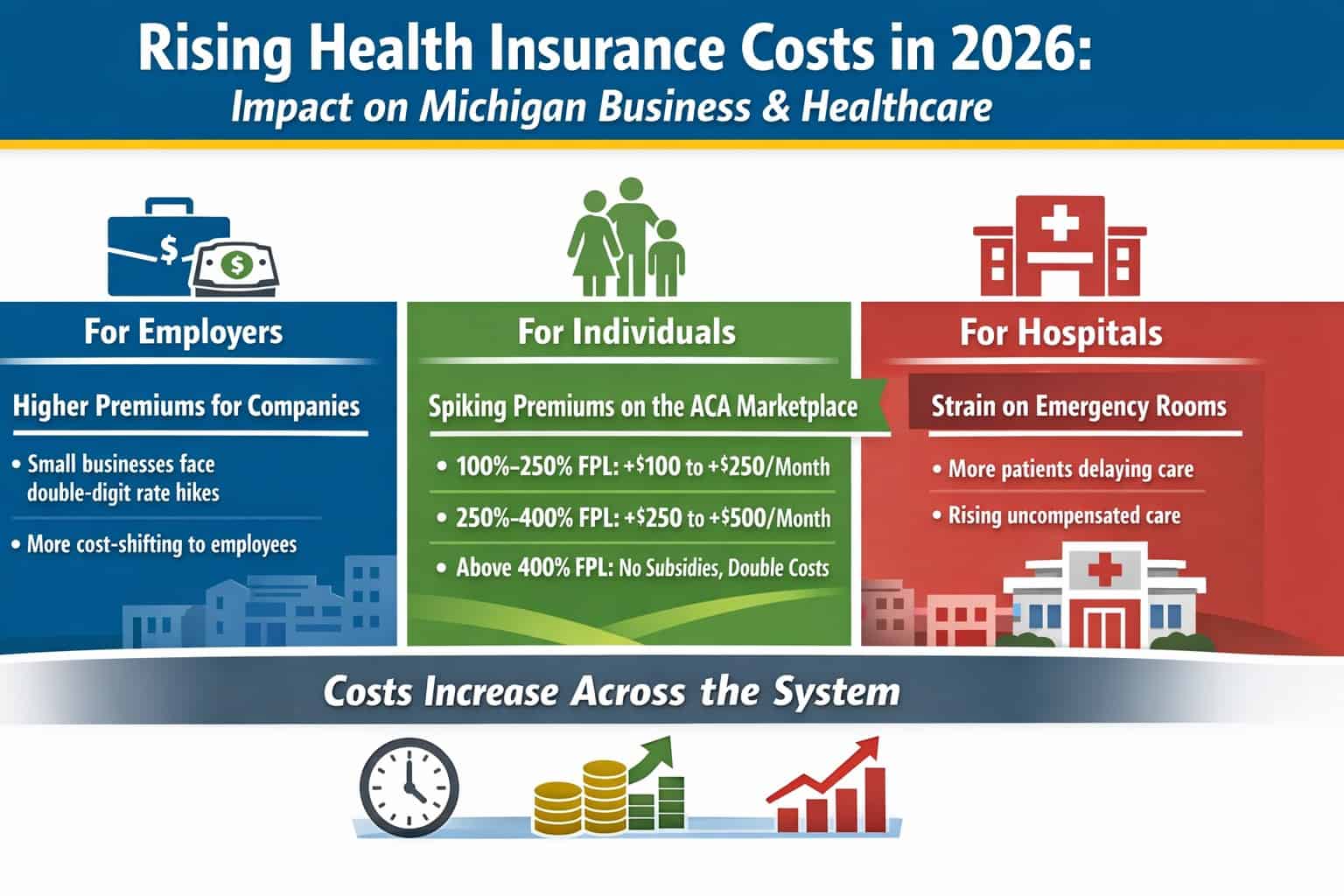
SWEDEN – Can a blood test based on the ratio of plasma phosphorylated tau 217 (p-tau217) relative to non–p-tau217 (expressed as percentage of p-tau217) combined with the amyloid-β 42 and amyloid-β 40 plasma ratio (the amyloid probability score 2 [APS2]) accurately identify Alzheimer disease in primary care and secondary care when prospectively applying predefined biomarker cutoff values?
Findings There were 1213 patients undergoing cognitive evaluation in primary or secondary care. The APS2 had high diagnostic accuracy (range, 88%-92%) for detecting Alzheimer disease pathology in both primary and secondary care. Dementia specialists identified clinical Alzheimer disease with a diagnostic accuracy of 73% vs 91% using the APS2 and primary care physicians had a diagnostic accuracy of 61% vs 91% using the APS2.
Meaning This blood test (the APS2) had high diagnostic accuracy for identifying Alzheimer disease among individuals with cognitive symptoms in primary and secondary care, providing superior performance compared with the diagnostic accuracy after standard clinical evaluation (not using Alzheimer disease biomarkers).
Abstract Importance An accurate blood test for Alzheimer disease (AD) could streamline the diagnostic workup and treatment of AD.
Objective To prospectively evaluate a clinically available AD blood test in primary care and secondary care using predefined biomarker cutoff values.
Design, Setting, and Participants There were 1213 patients undergoing clinical evaluation due to cognitive symptoms who were examined between February 2020 and January 2024 in Sweden. The biomarker cutoff values had been established in an independent cohort and were applied to a primary care cohort (n = 307) and a secondary care cohort (n = 300); 1 plasma sample per patient was analyzed as part of a single batch for each cohort. The blood test was then evaluated prospectively in the primary care cohort (n = 208) and in the secondary care cohort (n = 398); 1 plasma sample per patient was sent for analysis within 2 weeks of collection.
Exposure Blood tests based on plasma analyses by mass spectrometry to determine the ratio of plasma phosphorylated tau 217 (p-tau217) to non–p-tau217 (expressed as percentage of p-tau217) alone and when combined with the amyloid-β 42 and amyloid-β 40 (Aβ42:Aβ40) plasma ratio (the amyloid probability score 2 [APS2]).
Read more at JAMA